MAGNETISATION AND MAGNETIC INTENSITY
`color{blue} ✍️` The earth abounds with a bewildering variety of elements and compounds. In addition, we have been synthesising new alloys, compounds and even elements.
`color {blue}{➢➢}` One would like to classify the magnetic properties of these substances. In the present section, we define and explain certain terms which will help us to carry out this exercise.
`color{blue} ✍️` We have seen that a circulating electron in an atom has a magnetic moment. In a bulk material, these moments add up vectorially and they can give a net magnetic moment which is non-zero. We define magnetisation `color{blue}(M)` of a sample to be equal to its net magnetic moment per unit volume:
`color {blue}{➢➢}` `color{blue}(M)` is a vector with dimensions `color{blue}(L^(-1) A)` and is measured in a units of `color{blue}(A m^(–1)).`
`color {blue}{➢➢}` Consider a long solenoid of `color{blue}(n)` turns per unit length and carrying a current `color{blue}(I)`. The magnetic field in the interior of the solenoid was shown to be given by
`color {blue}{➢➢}` If the interior of the solenoid is filled with a material with non-zero magnetisation, the field inside the solenoid will be greater than `color{blue}(B_0).` The net `color{blue}(B)` field in the interior of the solenoid may be expressed as
`color {blue}{➢➢}` where `color{blue}(B_m)` is the field contributed by the material core. It turns out that this additional field `color{blue}(B_m)` is proportional to the magnetisation `color{blue}(M)` of the material and is expressed as
`color {blue}{➢➢}` where `color{blue}(μ_0)` is the same constant (permeability of vacuum) that appears in
Biot-Savart’s law. It is convenient to introduce another vector field `color{blue}(H)`, called the magnetic intensity, which is defined by
`color {blue}{➢➢}` where `color{blue}(H)` has the same dimensions as `color{blue}(M)` and is measured in units of `color{blue}(A m^(–1))`. Thus, the total magnetic field `color{blue}(B)` is written as
`color {blue}{➢➢}` We repeat our defining procedure. We have partitioned the contribution to the total magnetic field inside the sample into two parts: one, due to external factors such as the current in the solenoid. This is represented by `color{blue}(H)`.
`color {blue}{➢➢}` The other is due to the specific nature of the magnetic material, namely `color{blue}(M).` The latter quantity can be influenced by external factors. This influence is mathematically expressed as
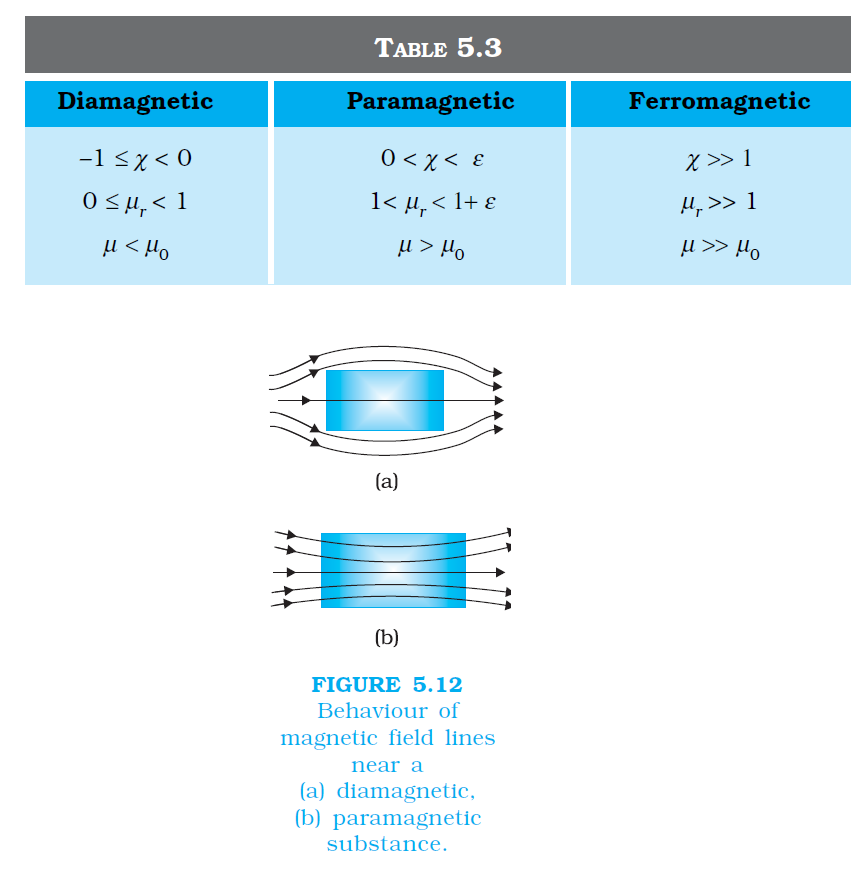
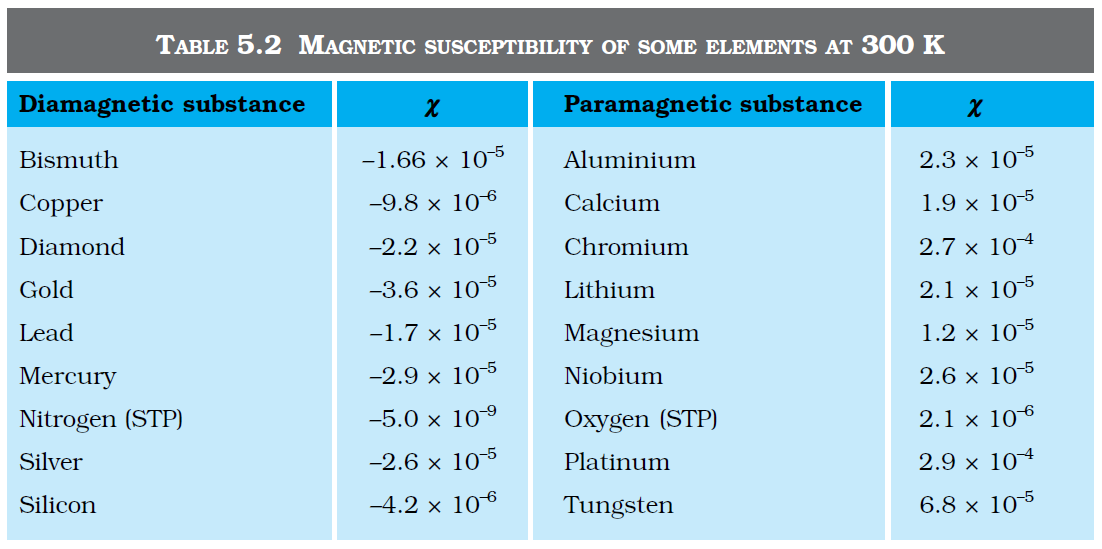
`color {blue}{➢➢}` where `color{blue}(χ) ,` a dimensionless quantity, is appropriately called the magnetic susceptibility. It is a measure of how a magnetic material responds to an external field. Table 5.2 lists `color{blue}(χ)` for some elements. It is small and positive for materials, which are called paramagnetic.
`color {blue}{➢➢}`It is small and negative for materials, which are termed diamagnetic. In the latter case `color{blue}(M)` and `color{blue}(H)` are opposite in direction. From Eqs. (5.16) and (5.17) we obtain,
`color{blue}(= mu_0mu_rH)`
`color {blue}{➢➢}`where `color{blue}(μ_r= 1 + χ,)` is a dimensionless quantity called the relative magnetic permeability of the substance. It is the analog of the dielectric constant in electrostatics. The magnetic permeability of the substance is `color{blue}(μ)` and it has the same dimensions and units as `color{blue}(μ_0)`.
`color{blue}(mu=mu_0 mu_r = mu_0 (1+χ))`
`color {blue}{➢➢}`The three quantities `color{blue}(χ, μ_r)` and `color{blue}(μ)` are interrelated and only one of them is independent. Given one, the other two may be easily determined.

`color {blue}{➢➢}` One would like to classify the magnetic properties of these substances. In the present section, we define and explain certain terms which will help us to carry out this exercise.
`color{blue} ✍️` We have seen that a circulating electron in an atom has a magnetic moment. In a bulk material, these moments add up vectorially and they can give a net magnetic moment which is non-zero. We define magnetisation `color{blue}(M)` of a sample to be equal to its net magnetic moment per unit volume:
`color{blue}(M = (m_(n e t))/V)`
.................(5.11)`color {blue}{➢➢}` `color{blue}(M)` is a vector with dimensions `color{blue}(L^(-1) A)` and is measured in a units of `color{blue}(A m^(–1)).`
`color {blue}{➢➢}` Consider a long solenoid of `color{blue}(n)` turns per unit length and carrying a current `color{blue}(I)`. The magnetic field in the interior of the solenoid was shown to be given by
`color{blue}(B_0 = mu_0 nI)`
.............(5.12)`color {blue}{➢➢}` If the interior of the solenoid is filled with a material with non-zero magnetisation, the field inside the solenoid will be greater than `color{blue}(B_0).` The net `color{blue}(B)` field in the interior of the solenoid may be expressed as
`color{blue}(B = B_0 + B_m)`
...........(5.13)`color {blue}{➢➢}` where `color{blue}(B_m)` is the field contributed by the material core. It turns out that this additional field `color{blue}(B_m)` is proportional to the magnetisation `color{blue}(M)` of the material and is expressed as
`color{blue}(B_m = μ_0 M)`
..........(5.14)`color {blue}{➢➢}` where `color{blue}(μ_0)` is the same constant (permeability of vacuum) that appears in
Biot-Savart’s law. It is convenient to introduce another vector field `color{blue}(H)`, called the magnetic intensity, which is defined by
`color{blue}(H= B/(mu_0) -M)`
..............(5.15)`color {blue}{➢➢}` where `color{blue}(H)` has the same dimensions as `color{blue}(M)` and is measured in units of `color{blue}(A m^(–1))`. Thus, the total magnetic field `color{blue}(B)` is written as
`color{blue}(B = mu_0(H+M))`
............(5.16)`color {blue}{➢➢}` We repeat our defining procedure. We have partitioned the contribution to the total magnetic field inside the sample into two parts: one, due to external factors such as the current in the solenoid. This is represented by `color{blue}(H)`.
`color {blue}{➢➢}` The other is due to the specific nature of the magnetic material, namely `color{blue}(M).` The latter quantity can be influenced by external factors. This influence is mathematically expressed as
`color{blue}(M = χ H)`
.....................(5.17)`color {blue}{➢➢}` where `color{blue}(χ) ,` a dimensionless quantity, is appropriately called the magnetic susceptibility. It is a measure of how a magnetic material responds to an external field. Table 5.2 lists `color{blue}(χ)` for some elements. It is small and positive for materials, which are called paramagnetic.
`color {blue}{➢➢}`It is small and negative for materials, which are termed diamagnetic. In the latter case `color{blue}(M)` and `color{blue}(H)` are opposite in direction. From Eqs. (5.16) and (5.17) we obtain,
`color{blue}(B=mu_0 (1+χ)H)`
...................(5.18)`color{blue}(= mu_0mu_rH)`
`color{blue}(= muH)`
....................(5.19)`color {blue}{➢➢}`where `color{blue}(μ_r= 1 + χ,)` is a dimensionless quantity called the relative magnetic permeability of the substance. It is the analog of the dielectric constant in electrostatics. The magnetic permeability of the substance is `color{blue}(μ)` and it has the same dimensions and units as `color{blue}(μ_0)`.
`color{blue}(mu=mu_0 mu_r = mu_0 (1+χ))`
`color {blue}{➢➢}`The three quantities `color{blue}(χ, μ_r)` and `color{blue}(μ)` are interrelated and only one of them is independent. Given one, the other two may be easily determined.